Tau is a highly soluble protein that normally stabilizes microtubules, commonly referred to as Microtubule-Associated Proteins (MAPs), primarily in neurons of the central nervous system (CNS) and to a much lesser extent expressed in astrocytes and oligodentrocytes. Hyperphosphorylation of the Tau protein (i.e., Tau inclusions or pTau) can result in the self-assembly of tangles of paired helical filaments (PHFs) and straight filaments, which are involved in the pathogenesis of Alzheimer’s disease (AD) and other related tauopathies. In AD brain tissue, all of the six isoforms of Tau are present in an often hyperphosphorylated state in PHFs. When Tau becomes misfolded, this otherwise very soluble protein can form extremely insoluble aggregates that contribute to a number of neurodegenerative tauopathies like AD. In addition, due to the observation of how Tau may be released extracellularly by an exosome-based mechanism and potentially spread in AD, it suggests that Tau has some similarities to prion proteins.
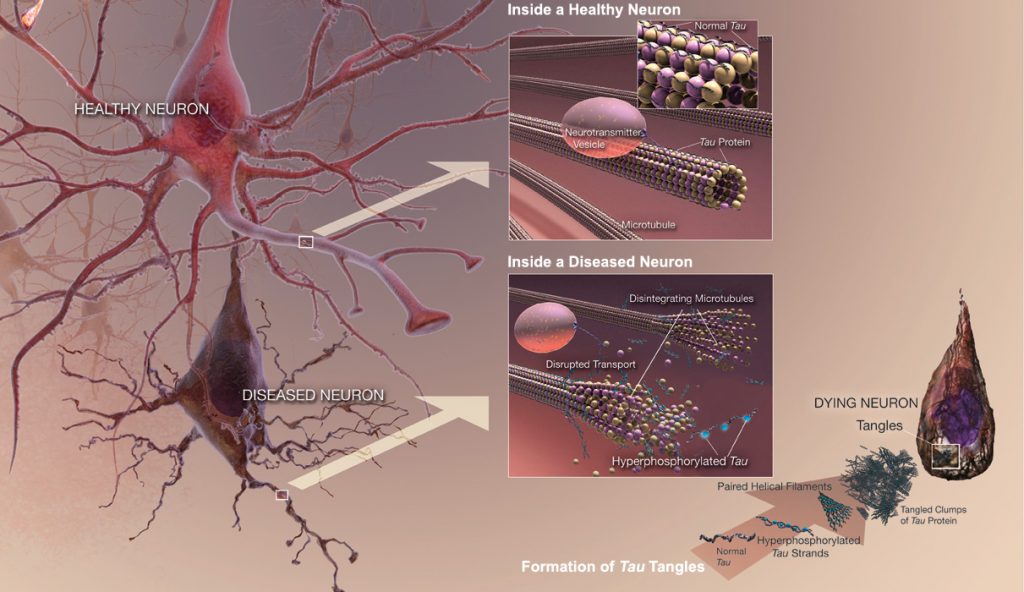 Unlike beta-amyloid (Aβ), Tau is a much larger protein with multiple epitopes that can possibly be hyperphosphorylated, and thereby multiple sites for prospective therapeutic intervention. INT has identified three separate monoclonal antibodies (mAbs) against three key and separate epitopes of the Tau protein that can potentially serve as passive immunotherapeutic strategies to reduce the cognitive decline (i.e., dementia or memory impairment) and pathological deficits observed not only in AD, but other neurodegenerative tauopathies listed below.
Unlike beta-amyloid (Aβ), Tau is a much larger protein with multiple epitopes that can possibly be hyperphosphorylated, and thereby multiple sites for prospective therapeutic intervention. INT has identified three separate monoclonal antibodies (mAbs) against three key and separate epitopes of the Tau protein that can potentially serve as passive immunotherapeutic strategies to reduce the cognitive decline (i.e., dementia or memory impairment) and pathological deficits observed not only in AD, but other neurodegenerative tauopathies listed below.

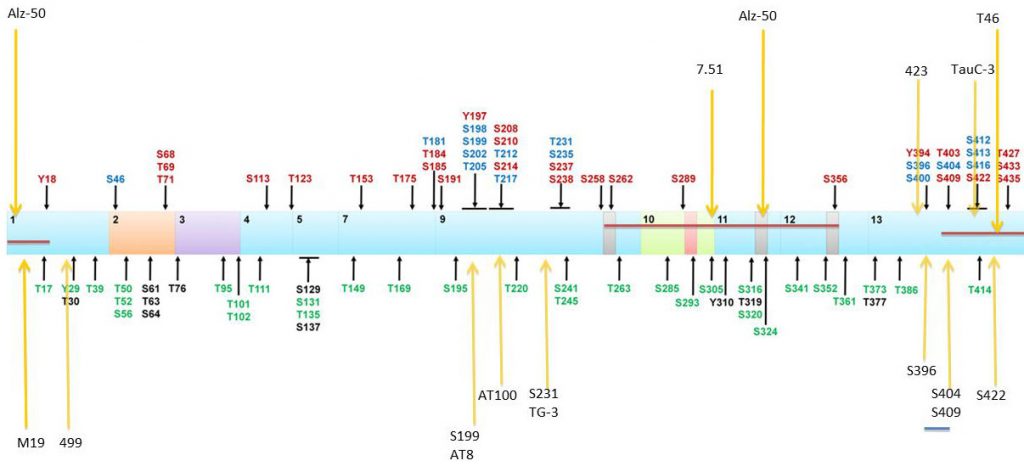 Location of Tau phosphorylation sites and epitopes for Tau antibodies. Multiple amino acids are phosphorylated with some of those observed in AD brain (red), normal brain (green), and both normal and AD brains (blue). Putative phosphorylation sites that have not yet been demonstrated in vitro or in vivo (black). Localization of antibody epitopes are indicated with arrows.
Location of Tau phosphorylation sites and epitopes for Tau antibodies. Multiple amino acids are phosphorylated with some of those observed in AD brain (red), normal brain (green), and both normal and AD brains (blue). Putative phosphorylation sites that have not yet been demonstrated in vitro or in vivo (black). Localization of antibody epitopes are indicated with arrows.